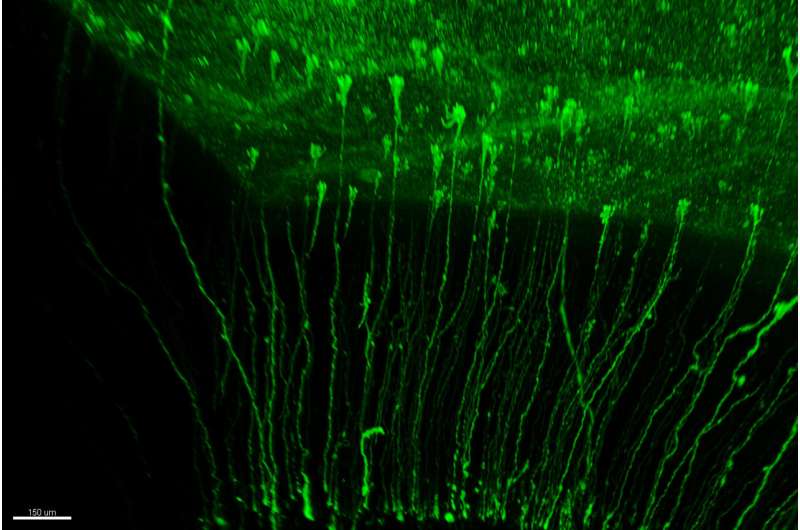
by admin | Apr 12, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions
by Elena Garrido, Miguel Hernandez University of Elche – MedXpress – The development of the cerebral cortex largely depends on the stem cells responsible for generating neurons, known as radial glial cells. Until now, it was believed that these stem cells...

by admin | Apr 10, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions
by Jen Brogan – PharmaTimes – Researchers from King’s College London’s (KCL) Comprehensive Cancer Centre have identified a key mechanism that governs how bone marrow stem cells work, which could potentially lead to new therapeutic pathways. The findings...
by admin | Apr 9, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions
by Susan Buckles – Mayo Clinic – Chimeric antigen receptor-T cell therapy (CAR-T cell therapy) could provide a revolutionary approach to organ transplantation for patients who are hard to match and susceptible to rejection, Mayo Clinic researchers...

by admin | Apr 5, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions, Video
By Mason Leib – ABC News – Good Morning America – A man who was paralyzed from the neck down after a surfing accident seven years ago is now able to stand and walk on his own, thanks in part to a potentially groundbreaking stem cell treatment. Chris...
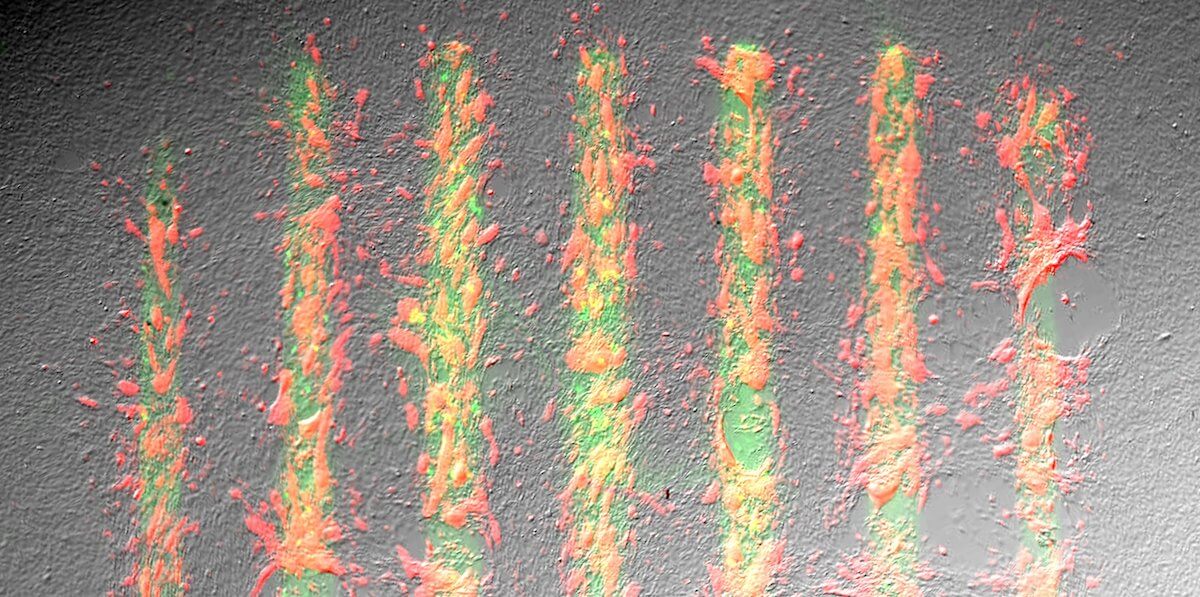
by admin | Apr 3, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions
by Greta Harrison – USC School of Engineering – USC Viterbi and Keck USC researchers, aided by Chan Zuckerberg Initiative grant, seek to control how cells grow into tissue, with game-changing applications in neuromuscular diseases and more. t may sound...
by admin | Mar 29, 2024 | Bernie Siegel’s WORLD STEM CELL SUMMIT BLOG, News and Opinions
By University of Galway – Medical Xpress – Neuroscientists at the University of Galway have made an exciting discovery that could revolutionize stem cell-based brain repair therapy for Parkinson’s disease. Parkinson’s is a neurodegenerative...







